TMJ Concepts
patient-specific implants
Market leading. Always evolving.
Overview
You’ve made us the market leader in craniomaxillofacial surgery, and you’ve made our TMJ Concepts patient-specific implant the gold standard in TMJ reconstruction. Built to spec from each patient's anatomy, our customized implants - combined with your expertise - can help create a brighter patient future with potentially less pain, more function, enhanced appearance and new-found hope.1
78% of surgeons prefer patient-specific TMJ implants over off-the-shelf4
Reasons you prefer patient-specific implants1,2,4
• Ease-of-use
• Improved outcomes and quality of life
• Long-term reliability
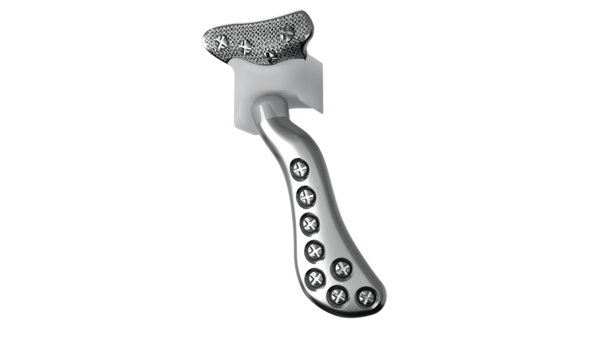
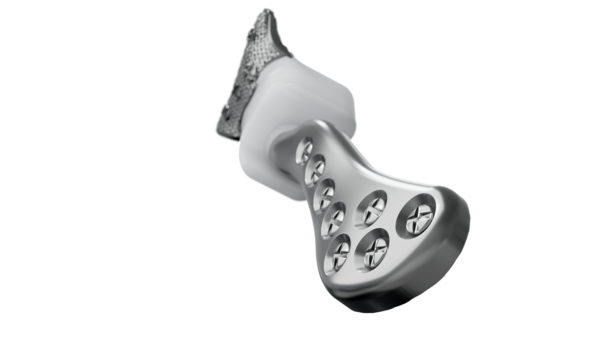
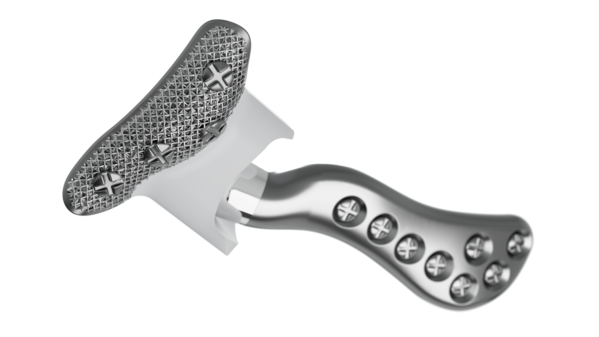
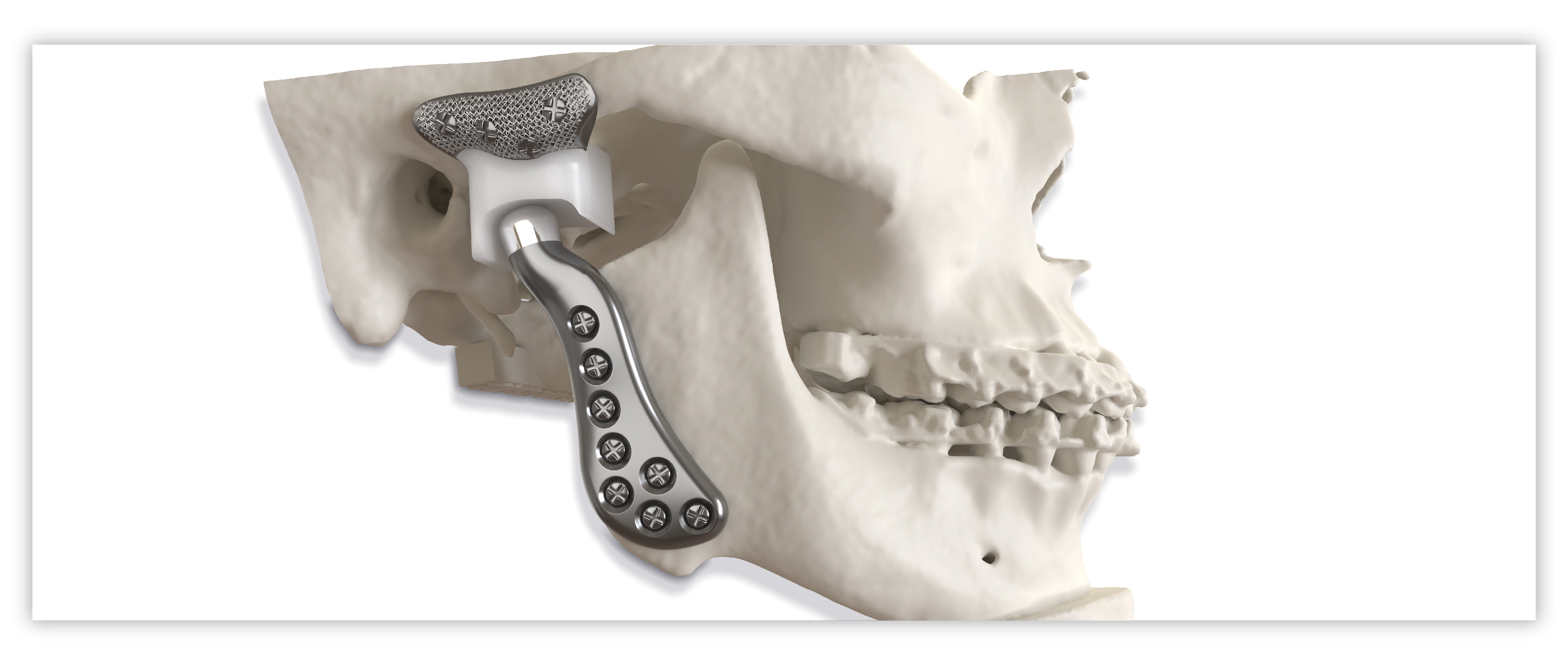
Implant components
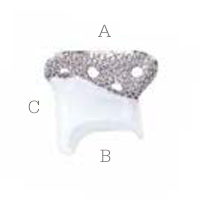
Glenoid fossa component
No two are the same;
distinct designs measured
and fabricated per patient
A Unalloyed titanium mesh backing
B Ultra-high molecular weight
polyethylene
C Industry-exclusive posterior
lip design
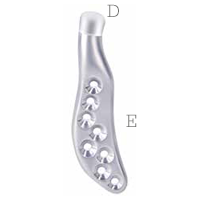
Mandibular component
Individually designed for
unique patient anatomy
D Cobalt-chromium-molybdenum alloy condylar head
E Titanium alloy body
True original. Genuine difference.
Precision fit and function
- Individualized implants address each patient’s anatomical idiosyncrasies
- Fossa titanium mesh backing for secure screw fixation
- Lip design of the fossa helps to minimize the possibility of posterior dislocation
- Enhances preoperative planning and precision
Total assurance
- Evidence-based design backed by positive patient outcomes1
- Made of trusted, clinically-proven materials2
- Helps build patient confidence knowing implant is personalized
- Brought to you by the market leader in CMF fixation
Simplified workflow
- Helps streamline perioperative workflow since implants are tailored per patient
- Less recontouring of the bone needed3
Case initiation
All case initiation and CT uploads are now accepted through the iD Portal. Please contact moc.rekyrts@stnalpmidezimotsuCFMC or your local Stryker sales representative with questions.
Contraindications
The TMJ Concepts Patient-Fitted TMJ Reconstruction Prosthesis System should not be used for patients with one or more of the following conditions:
- Active or suspected infections in or about the implantation site
- Uncontrollable masticatory muscle hyperfunction (clenching or grinding) which may lead to overload and loosening of screws
- Known allergy to any of the component materials
Clinical data
A total of 279 patients (465 joints) were enrolled in a post-approval study in which clinical data was collected both pre-operatively (month 0) and post-operatively at various follow-up intervals out to 5 years (month 60). Based on previous clinical studies of TMJ patients, it was anticipated that a large number would become lost to follow-up. It was desired to have a cohort of at least 100 patients remaining at the 5-year evaluation time point, therefore a significantly larger number of patients were enrolled in the study. Clinical data was obtained out to 5 years on a final cohort of 128 patients (204 joints). Pre-operative data and post-operative follow-up data were collected using a standardized data collection format. Subjective data related to pain, function of the lower jaw, and diet were obtained using a 55mm length visual analogue scale. The pain scale ranged from "no pain" at 0mm to "severest pain" at 55mm. The function scale ranged from "no loss" at 0mm to "cannot function" at 55mm. The diet scale ranged from "no restriction" at 0mm to "liquids only" at 55mm. Subjective data was also collected by asking each patient how their current quality of life compared to before they received their TMJ implants. Objective measurements of mandibular range of motion were made directly on the patients. These measurements, recorded in millimeters, included maximum interincisal opening and left and right excursion. Results are shown for only the month 0 and month 60 evaluation time points as clinical data was not available for each patient at every intermediate follow-up interval. These clinical data show a statistically significant decrease in pain, increase in function, decrease in diet restrictions, and increase in maximum interincisal opening. A summary of the quality of life responses at month 60 is also shown.
Pain Measurement Improvement at 5 Years
(scale: 0mm = "no pain" to 55mm = "severest pain")
|
Month |
Mean (mm) |
S.D.(mm) |
|
0 |
39.0 |
13.4 |
|
60 |
18.3 |
15.9 |
Function Measurement Improvement at 5 Years
(scale: 0mm = "no loss" to 55mm = "cannot function")
|
Month |
Mean (mm) |
S.D.(mm) |
|
0 |
36.4 |
13.0 |
|
60 |
17.9 |
13.8 |
Diet Measurement Improvement at 5 Years
(scale: 0mm = "no restriction" to 55mm = "liquids only")
|
Month |
Mean (mm) |
S.D.(mm) |
|
0 |
32.4 |
14.2 |
|
60 |
14.7 |
13.4 |
MIO Measurement Increase at 5 Years
|
Month |
Mean (mm) |
S.D.(mm) |
|
0 |
25.0 |
11.2 |
|
60 |
33.4 |
9.2 |
Summary of Quality of Life Responses at 5 Years How does your current quality of life compare to before you received your TMJ implants?
|
Response |
Percentage of Patients |
|
Much Better |
52.3% |
|
Better |
25.8% |
|
Same |
7.8% |
|
Worse |
12.5% |
|
Much Worse |
1.6% |
Several of the patients enrolled in the post-approval study that were not included in the final cohort of 128 patients had adverse events reported prior to their becoming lost to follow up or being removed from
the study for another reason. The adverse event data presented below includes events reported for any of the 279 initially enrolled patients.
These types of adverse events and the rate at which they occurred as well as the quality of life responses shown above are not unexpected in this compromised patient population with many previous surgeries involving failed tissue grafts and/or failed implants from other manufacturers which may leave behind material particulates.
|
Adverse Events Resulting in Additional Surgery |
||||
|
Category |
Patients (n=279) |
Joints (n=465) |
||
|
No. |
% |
No. |
% |
|
|
Chronic or recurring pain and/or swelling |
4 |
1.4% |
4 |
0.9% |
|
Infection |
3 |
1.1% |
3 |
0.6% |
|
Dislocation of implant components |
2 |
0.7% |
3 |
0.6% |
|
Perforation or dehiscence of surrounding tissues |
2 |
0.7% |
2 |
0.4% |
|
Loosening |
2 |
0.7% |
2 |
0.4% |
|
Material sensitivity (reaction to implant components) |
1 |
0.4% |
2 |
0.4% |
|
Malocclusion |
1 |
0.4% |
1 |
0.2% |
|
Total |
15 |
5.4% |
17 |
3.7% |
Clinical evidence
Abstract
Currently only two alloplastic temporomandibular joint (TMJ) total joint replacement (TJR) systems are available in the United States. The aim of this study
was to define variables that determine whether a Biomet stock prosthesis could have been used to reconstruct a TMJ previously reconstructed with a TMJ Concepts patient-fitted prosthesis. All of the TMJ Concepts prostheses placed between 2010 and 2018 at the University of Texas – Health at San Antonio were analyzed retrospectively. There were 128 cases (241 joints) with intact stereolithographic models analyzed for successful adaptation of the Biomet stock TMJ prosthesis. Anatomical, demographic, etiological, and perioperative data were gathered for each joint to investigate possible causes of failure of stock adaptation. The majority of joints, 74% (178/241), could have had a stock prosthesis adapt. All joints with ≥40 mm gap arthroplasty failed stock prosthesis adaptation. Only 50% (32/64) of the joints with at least one previous open TMJ surgery and 60% (58/96) of the joints with concomitant orthognathic surgery could have had a stock TMJ prosthesis. The stock prosthesis could not be adapted for any of the patients requiring TMJ replacement for congenital disorders or those requiring TMJ salvage. Overall, the majority of cases treated with a patient-specific TMJ TJR could have been treated with a stock prosthesis.
Abstract
Purpose: To evaluate subjective and objective outcomes of patients receiving Techmedica (currently TMJ Concepts) patient-fitted temporomandibular joint (TMJ) total joint replacement (TJR) devices after 19 to 24 years of service.
Patients and methods: This prospective cohort study evaluated 111 patients operated on by 2 surgeons using Techmedica (Camarillo, CA) patient-fitted TMJ TJR devices from November 1989 to July 1993. Patients were evaluated before surgery and at least 19 years after surgery. Subjective evaluations used standard forms and questions with a Likert scale for 1) TMJ pain (0, no pain; 10, worst pain imaginable), 2) jaw function (0, normal function; 10, no movement), 3) diet (0, no restriction; 10, liquid only), and 4) quality of life (QoL; improved, the same, or worse). Objective assessment measured maximum incisal opening (MIO). Comparison analysis of presurgical and longest follow-up data used nonparametric Mann-Whitney and Wilcoxon signed rank tests. Spearman correlations evaluated the number of prior surgeries in relation to objective and subjective variables.
Results: Of the 111 patients, 56 (50.5%) could be contacted and had adequate records for inclusion in the study. Median follow-up was 21 years (interquartile range [IQR], 20 to 22 yr). Mean age at surgery was 38.6 years (standard deviation, 10 yr). Median number of previous TMJ surgeries was 3 (IQR, 4). Presurgical and longest follow-up data comparison showed statistically significant improvement (P < .001) for MIO, TMJ pain, jaw function, and diet. At longest follow-up, 48 patients reported improved QoL, 6 patients reported the same QoL, and 2 patients reported worse QoL. Spearman correlations showed that an increased number of previous surgeries resulted in lower levels of improvement for TMJ pain and MIO.
Conclusions: At a median of 21 years after surgery, the Techmedica/TMJ Concepts TJR continued to function well. More previous TMJ surgeries indicated a lesser degree of improvement. No devices were removed owing to material wear.
Abstract
Purpose: To measure and identify factors associated with treatment outcomes for patients with temporomandibular joint (TMJ) ankylosis treated with TMJ Concepts patient-fitted total joint prostheses and autogenous fat grafts.
Patients and methods: This retrospective cohort study evaluated records of patients with TMJ ankylosis from a single private practice, treated from 1992 to 2011, who met the following inclusion criteria: 1) radiographic evidence of bony ankylosis, 2) limited incisal opening, 3) minimum of 12 months' follow-up, and 4) treatment with TMJ Concepts (Ventura, CA)/Techmedica (Camarillo, CA) total joint prostheses and fat grafts. For each patient, the number of previous TMJ surgical procedures, as well as the estimated age of ankylosis onset, age at surgery, and length of postoperative follow-up, was recorded. Subjective evaluations were made with Likert-like scales (from 0 to 10) for 1) TMJ pain, 2) headache and facial pain, 3) jaw function, 4) diet, and 5) disability. Objective evaluations included maximal incisal opening and excursion movements. Nonparametric statistics were used for analysis.
Results: There were 32 patients (22 female and 10 male patients) with 48 ankylosed TMJs (16 bilateral and 16 unilateral) in this study, with a mean age of 39 years (range, 11 to 68 years), 2 or more previous TMJ surgical procedures in 69%, and a mean follow-up period of 68 months (range, 12 to 168 months). Trauma was the major etiology of TMJ ankylosis, occurring in 17 of 32 patients (53%). The following improvements occurred: The median value for TMJ pain changed from 8.0 preoperatively to 1.5 at longest follow-up; headache, from 8 to 3.5; facial pain, from 8 to 4; jaw function, from 8 to 2.5; diet, from 7 to 3; and disability, from 7 to 1.5. The median incisal opening was 14.5 mm (interquartile range, 6.3 to 20 mm) preoperatively and 35 mm (interquartile range, 30 to 40 mm) at longest follow-up. The median left lateral excursion improved from 0.5 to 2 mm, and the median right lateral excursion improved from 1 to 1.3 mm. All of these improvements were highly significant (P < .001, Wilcoxon tests). Equally favorable outcomes were found in patients with 12 to 48 months of maximal follow-up and patients with more than 48 months of maximal follow-up.
Conclusions: The treatment of TMJ ankylosis with the TMJ Concepts patient-fitted total joint prosthesis in combination with fat grafting around the articulation area of the prosthesis is a viable and predictable method for improving pain levels, function, and quality of life, as well as prevention of reankylosis of the TMJ.
Abstract
Total replacement of the temporomandibular joint (TMJ) is increasingly accepted as the gold standard for reconstruction of irreparably damaged or ankylosed joints. The TMJ Concepts system (TMJ Concepts, Ventura, USA) has the longest follow-up of the 2 systems used in the UK. A total of 74 patients had placement of TMJ Concepts prostheses. The primary diagnoses were degenerative disease, multiple previous operations, injury, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and ankylosis. Of these, 12 were revisions of previous replacements (3 after multiple operations). Over the year there was a significant mean (SD) reduction in pain score (10 cm visual analogue scale) from 72 (2.5) to 8 (1.7) (p<0.0001), and mean (SD) improvements in mouth opening from 22.4 mm (9.4) to 33.7 mm (6.2) (p<0.0001), and dietary consistency (10 cm analogue liquid 0 to solid 100) from 38 (23) to 93 (16) (p<0.0001). No patient had worse symptoms postoperatively. Joints in 2 patients failed because of biofilm infections. Two patients required blood transfusion and one required ligation of the external carotid artery. Five had perioperative dislocation, which responded to elastic intermaxillary fixation for one week. A total of 31 patients had partial, and 2 had total weakness of the facial nerve. All resolved fully except weakness of the temporal branch in one patient, which required brow lift. Total TMJ replacement gives good early improvements in function and pain with few complications. Of the 74 patients, 71 were very pleased to have had the procedure. One was dissatisfied despite complete pain relief and improvement in mouth opening from 3 to 30 mm, and 2 were ambivalent (one had infection, revision, and permanent weakness of the temporal branch of the facial nerve).
References
1. Wolford LM, Mercuri LG, Schneiderman ED, et al. Twenty-year follow-up study on a patient-fitted temporomandibular joint prosthesis: the Techmedica/TMJ Concepts device. J Oral and Maxillofacial Surgery. 2015;73(5):952-60.
2. TMJ Concepts Sterile Implant IFU
3. Brown ZL, Sarrami S, and Perez DE. Will they fit? Determinants of the adaptability of stock TMJ prostheses where custom TMJ prostheses were utilized. Int J Oral Maxillofacial Surgery. 2021; 50(2):220-226.
4. 2020 TMJ pain market survey findings
5. Stryker data on file
6. Orthognathic surgery patient journey, qualitative research report, May 2022. Bauman Research & Consulting
FAC-WEB-1_Rev. None_34098